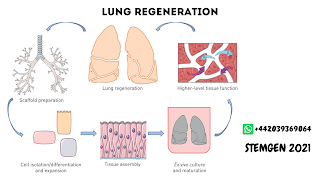
What is scaffolding in regenerative medicine?
Stem cells are self-renewing cells
that can be differentiated into other cell types. Conventional in vitro models
for studying stem cells differentiation are usually preformed in
two-dimensional (2D) cultures. The design of three-dimensional (3D) in vitro
models which ideally are supposed to mimic the in vivo stem cells
microenvironment is potentially useful for inducing stem cell derived tissue
formation. Biodegradable scaffolds play an important role in creating a 3D
environment to induce tissue formation. The application of scaffolding
materials together with stem cell technologies are believed to hold enormous
potential for tissue regeneration. In this review, we provide an overview of
application of tissue engineered scaffolds and stem cells for the development
of stem cell-based engineered tissue replacements. In particular, we focus on
bone marrow stem cells (BMSCs) and mesenchymal stem cell (MSCs) due to their extensive
clinical applications.
Stem cells are primitive cells found
in many multi-cellular organisms and possess self-renewal and potency
abilities. Self-renewal is that characteristic of stem cells that maintains
them in numerous cell cycle divisions, while potency defines the
differentiation capability of stem cells into mature cell types. Mammalian stem
cells are categorized based on the source they are derived from: embryonic stem
(ES) cells, derived from blastocysts, and adult stem cells, found in adult
tissues
Cell proliferation in 3-D scaffold,
needs oxygen and nutrition supply. Therefore, the 3-D scaffold materials should
provide such an environment for cells. The artificial scaffolds formed by
self-assembling molecules not only provide suitable support for cell
proliferation but also serve as a medium through which diffusion of soluble
factors and migration of cells can occur. The result of the cell attachment and
proliferation revealed that diffusion of nutrients, bioactive factors, and
oxygen through these highly hydrated networks is sufficient for survival of
large numbers of cells for extended periods of time.
The 3D scaffolds are capable of
differentiating a single progenitor cell population into particular lineage
either due to bulk incorporation of soluble factors within the scaffolds or due
to exogenous delivery of chemicals, hormones, and growth factors in culture
medium. Therefore, design of patterned scaffolds with the ability to develop multiple
lineages and hybrid organ structures could provide promising alternatives.
Metal scaffolds like titanium are
bio-compatible and suitable for hard-tissue applications, such as the growth
and differentiation of rat dental pulp progenitor cells into odontoblast-like
cells. In order to improve their efficacy, metal scaffolds can be covered with
biological compounds, like titanium fibers pre-coated with ECM components that
support the osteogenic differentiation of rats’ BMSCs.
Another type of scaffolds is made of
organic materials that provide a bio-mimetic environment for stem cells. Human
BMSCs regenerate bone in marine sponge skeletons, cartilage in silk fibroin
scaffolds, and adipose tissue in gelatin. To provide mechanical strength,
biological agents influencing stem cell fate could be added to the scaffold’s
compounds. Marine sponge skeletons, for example, contain these cell adhesion
proteins: fibronectin, collagen and gelatin.
The application of scaffolding
materials together with stem cell technologies are believed to hold enormous
potential for tissue regeneration. In this review, we provide an overview of
application of tissue engineered scaffolds and stem cells for the development
of stem cell-based engineered tissue replacements. In particular, we focus on
bone marrow stem cells (BMSCs) and mesenchymal stem cell (MSCs) due to their
extensive clinical applications.


Comments
Post a Comment